Course Description
The Statistical Quality Control and Method Validation/Verification (QCMV2) course is focused on quantitative testing, including CD4 testing, with examples of biochemistry and hematology. This course provides participants with the crucial knowledge and tools to evaluate and monitor their quantitative tests.
With lecture recordings on the Moodle platform and Zoom-based interactive live webinars, participants will learn to:
- Select new methods and instruments with performance specifications that relate to "the intended use" of the procedure (ISO15189:2022, 7.3)
- Introduce new methods and instruments into laboratories by properly evaluating the procedures for “the intended use” (ISO15189:2022, 7.3.1)
- Design a robust internal QC program for all the tests (ISO15189:2022, 7.3.7.2)
- Assess on-going laboratory's accuracy through External Quality Assessment Schemes (EQAS) (ISO15189:2022, 7.3.7.3)
Target Audience
The target audience includes:
- Laboratory directors. managers, supervisors, or QC officers involved with conducting quantitative QC and method validation/verification.
- Senior laboratory staff involved in quantitative testing and method validation/ verification.
- Quality managers, project managers and mentors who are implementing QMS in laboratories.
- Auditors and assessors who have a technical background in clinical pathology.
- Those who conduct QMS training including statistical QC and validation/verification or involved in pre-service curriculum.
Course Format
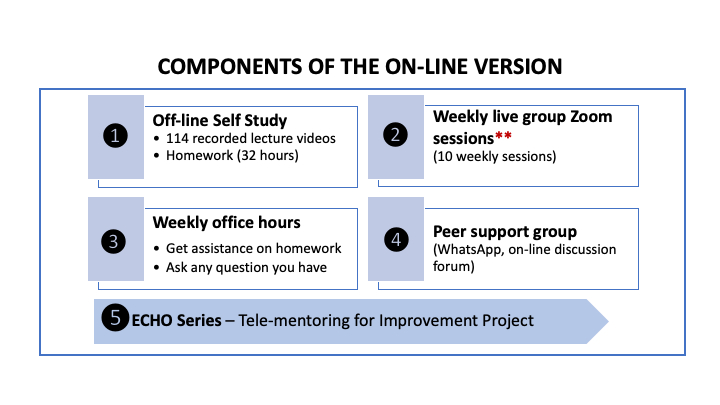
This course contains 10-week online learning followed by 4 ECHO sessions delivered over 2 weeks. This eLearning course uses a “flipped classroom” model (definition). Participants engage in self-study, learning from 100+ online lecture videos and completing weekly assignments, before they attend live Zoom-based sessions for debrief, discussion, and Q&A. The ECHO sessions allow the participants to work on their own laboratory's data and get mentoring from the facilitators.
Criteria for the Course Certificate
- Submission of weekly homework before deadlines
- Attendance – no more than 3 missed live sessions (i.e., webinars, office hours, ECHO sessions). If you miss a session, you must view the live session recording and submit evidence of having viewed the recording.
- Completion of weekly course evaluations
- A passing grade of 75 points on the post-test
Testimonials
QCMV eLearning 2024 Cohort
Now that I know how to do the right things, I consider myself a QCMV brand ambassador by not only teaching and mentoring colleagues but most importantly, walking the talk. This will tremendously improve the quality of the laboratory's results which will translate into quality patients' care. There will be no better testimony than the number of patients receiving high quality laboratory results as a result of skills and technical know-how gained from the QCMV Course.
Sanderson, Malawi
QCMV eLearning 2024 Cohort
The interactive nature of the course distinguishes it from other e-learning programs by giving you the opportunity to ask questions directly to the facilitators and be clarified. it also offers the opportunity to interact directly with participants from other labs around the world for experience sharing. If this course is completely applied, the frontline worker will be confident in the results produced and the laboratory will be contributing to proper diagnosis and treatment of patients.
I really appreciate this course.
Mbongko, Cameroon
QCMV eLearning 2024 Cohort
I recommend taking this course to all laboratory professionals because it:
- Goes along with a ‘simple words about the complex’ approach
- Includes exclusively important and essential information for routine work
- Is based on a thoroughly planned and thought program which is a great theory-practice combination
- Rewards you with fantastic atmosphere that contributes to professional growth
Iryna, Ukraine
QCMV eLearning 2024 Cohort
I found and experienced the QCMV course transformative! It hectic but extremely necessary. It is a world where your skills meet purpose. You find new colleagues, who share the same goals as you, you learn from the world on how to implement QC in your laboratories. With engaging lectures and practical exercises, it's a journey of growth and empowerment. Join me in unlocking our potential as scientists and making a difference.
Mbalenhle, South Africa
QCMV eLearning 2024 Cohort
I would recommend this course because it provides the laboratory with many statistical tools aligned to ensure the validity of examination results, as specified in the ISO 15189:2022 standard. I found this course very interesting, as it involves continuous learning and practicing exercises on worksheets, job aids, and handouts, aimed at understanding the objectives of the activities, restating the main ideas, and implementing them in the laboratory routine.
Janett, Peru
QCMV eLearning 2024 Cohort
The QCMV course is an extraordinary and useful one. In the laboratory, we used to carry out quality controls (internal and external) but we didn't have a lot of knowledge and mastery of certain performance criteria of a test or method. We also maked a limited interpretation of our QC results, method comparison, linearity, accuracy, and precision. Now because of this course, facilitated by highly competent experts, we know even more about how to ensure and improve the quality of our laboratory analysis results. We really thank to the organizers of this course and to all the facilitators.
Papa, Senegal
QCMV eLearning 2024 Cohort
Definitely, I would recommend QCMV course to my colleagues. Firstly, QCMV course provided me with a lot of important information about statistical quality control in the laboratory, validation/verification, PT, in addition, it also provided practical tools and strategies for improving processes in the laboratory, which I can and will directly apply in our laboratory to increase our measurements and research results reliability. QCMV course really helped me to improve my skills, I personally received a lot of information, so I will confidently recommend it to my colleagues, share and show in practice everything that I received in this course, how to correctly apply tools and rules, interpret the results obtained both inside the laboratory and results external quality assessment programs etc.
Baarinisa, Kyrgyz Republic
CONGRATULATIONS TO 2024 QCMV ELEARNING WINNERS!

Iryna Tanasiichuk
Ukraine

Christine Thomas
Trinidad and Tobago

Michael Kenyi Samuel Logoro
South Sudan

Janett Portilla
Peru

Sanderson Chilanga
Malawi
The Virtual Incentive Program is designed to promote timely attendance and submission of assignments, quality homework, active class participation, and outstanding contribution to shared learning. Participants earn virtual SLMTA dollars based on criteria. The winners (top earners) are awarded real, not virtual, prizes.
Scientific Committee
Below is a list of Scientific Committee members that contributed to the development of the SLMTA 3 e-learning course:
- Dr. Katy Yao, Centers for Disease Control and Prevention (CDC), USA
- Mr. Ashaba Davis, African Field Epidemiology Network (AFENET), Uganda
- Ms. Anna Murphy, Independent Consultant, USA
- Mr. Elde Mel Paladar, Adventist Health International and Loma Linda University, Malawi
- Mr. Monte Martin, Centers for Disease Control and Prevention (CDC), USA
- Ms. Janet Scholtz, QMS Consultant (Retired from National Health Laboratory Services, South Africa)
- Dr. Luciana Kohatsu, Centers for Disease Control and Prevention (CDC), USA

 Statistical Quality Control and Method Validation/Verification (QCMV2)
Statistical Quality Control and Method Validation/Verification (QCMV2)