About the Course
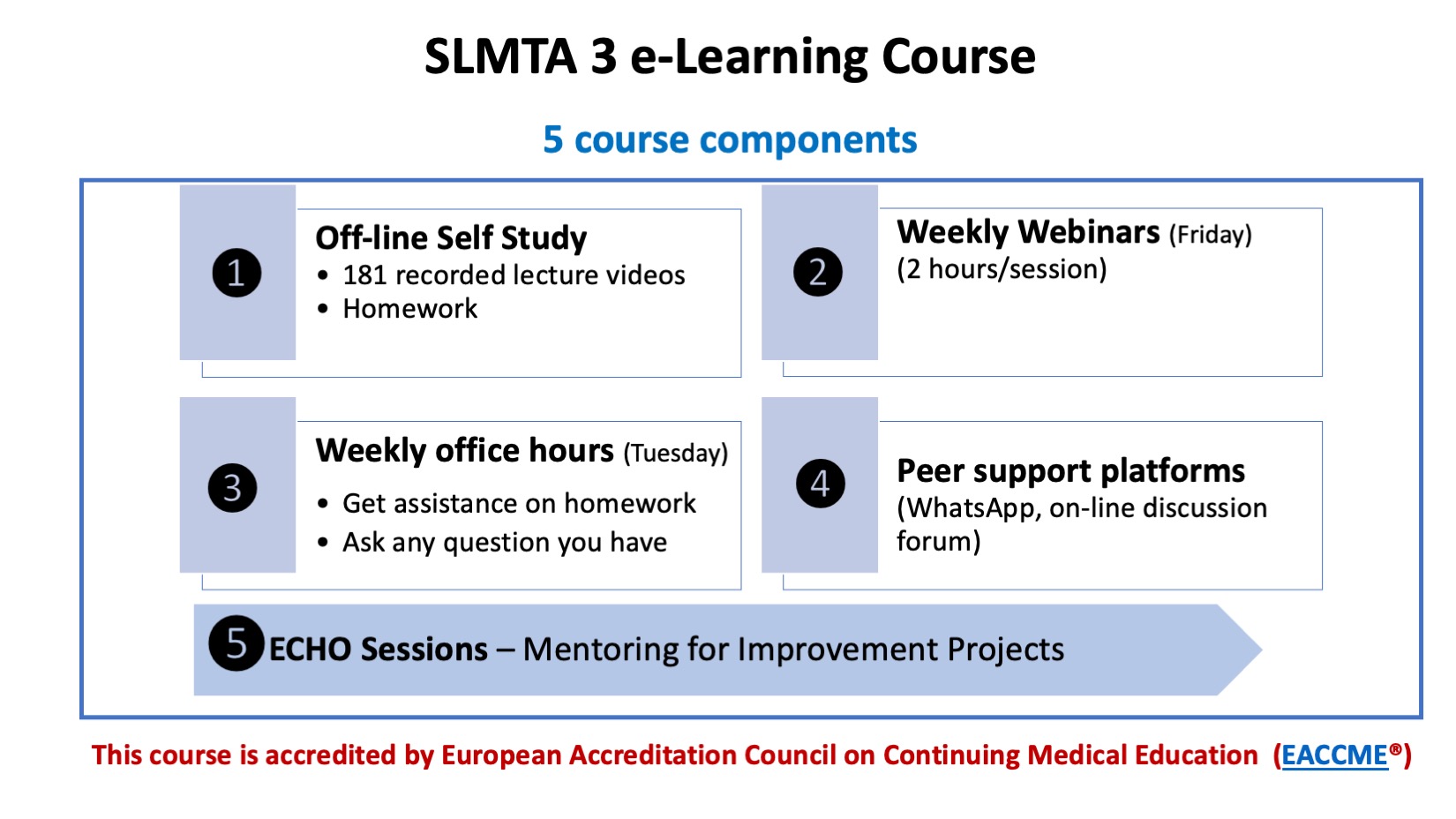
The SLMTA 3 curriculum is composed of four modules – QMS 1, QMS 2, QMS 3, and QMS4. Each module is further divided into sections and activities. When repurposing the classroom version of the curriculum for on-line delivery, the following 4 activities have been eliminated: Planning Improvement Projects – Master Class, Reporting Improvement Projects, Conducting a Site Visit, Redesigning The Floor Plan of Your Laboratory, and What’s Wrong with this Storeroom. Improvement project planning and implementation will be instead covered in the new ECHO sessions.
The on-line version includes an off-line/self-study component (lecture recordings and homework assignments) and an on-line/live component, as well as optional office hours and peer support discussion forum. The total time for completing the mandatory components of the on-line curriculum is 75 hours, as opposed to 86 hours of delivery time in the classroom based version.
COURSE OBJECTIVES
Participants will learn to:- Translate ISO15189 clauses into daily laboratory practice
- Recognize QMS as a whole and its interconnected processes
- Design and implement those processes for a cost-effective QMS that fulfills ISO15189 requirements
- Establish a practical QMS that enables laboratories to reach and sustain accreditation status
Criteria for obtaining the workshop certificate and CPD
- Submission of homework – 48 hours before the live session each week
- Attendance of live sessions – participants are allowed to miss up to 3 live sessions. However, they must view the recordings and complete certain tasks to show evidence of having viewed the live session recordings.
- Completion of course evaluation
SLMTA 3 Curriculum Overview Navigating the SLMTA 3 e-learning website
| Table of Contents | |
QMS 1 - MANAGEMENT RESPONSIBILITY
|
Launch |
QMS 2 - RESOURCE MANAGEMENT
|
Launch |
QMS 3 - PATH OF WORKFLOW
|
Launch |
QMS 4 - EVALUATION & CONTINUAL IMPROVEMENT
|
Launch |
Testimonials
SLMTA 3 eLearning Course – Cohort 5 (2024)
Thank you for a wonderful, life-changing experience. … If your lab is trying to get ISO 15189 accredited, it's easy to figure out exactly what you lack. There are checklists that will tell you how you will fail, and in great detail. That's how our lab used to approach this goal, and we didn't get very far. But if you want to learn how to fill those gaps--how to win--then take SLMTA.
Vladimir Sianghio
Philippines
SLMTA 3 eLearning Course – Cohort 5 (2024)
As the Deputy Manager of the HIV Reference Laboratory in South Sudan, an accredited lab under ISO 15189:2012, transitioning to the 2022 version, this course has been a game changer.
The course is incredibly practical and insightful, delivered by an amazing team. If you’re in laboratory management or quality assurance, the SLMTA course is a must! It’s the perfect opportunity to build solid foundations in lab quality management, prepare for ISO accreditation, and make a real impact on public health.
The content is top-notch, the instructors are top-tier, and the lessons are immediately applicable in your lab. You’ll leave feeling empowered and equipped to lead your team in QMS.
Robert Lubajo Zamba Lugor
South Sudan
SLMTA 3 eLearning Course – Cohort 5 (2024)
This course has been a challenging and transformative journey. Over the course of five months, I went through modules that helped me enhance existing processes and acquire new knowledge that I was previously unaware of, but now consider indispensable.
The SLMTA model will undoubtedly serve as an inspiration for us to develop national-level quality programs, enabling our laboratories to achieve and maintain accreditation status.
Mayrla Moniz
Brazil
SLMTA 3 eLearning Course – Cohort 5 (2024)
When I enrolled in the eLearning course I was excited and scared. Excited because it's a learning opportunity I wouldn't want to miss and scared because the agenda, homework and activities seemed packed and intense allowing no margin for inactivity. I must admit that though this journey is not a walk in the park, it has been a walk to remember and I will enroll over and over again if the opportunity comes. The course has lived and exceeded my expectations.
Ebenezer Benefo
Ghana
SLMTA 3 eLearning Course – Cohort 5 (2024)
The updated ISO 15189:2022 emphasizes risk management, continuous improvement, and patient-centered care—all of which were covered in practical and meaningful ways throughout the course. SLMTA provided me with actionable strategies to improve quality, streamline workflows, and ensure reliable services.
If you are actively involved in laboratory quality management or your goal is to meet ISO 15189:2022 requirements, the SLMTA course is exactly what you need. It’s not just another training—it’s a transformation.
This course equips you with practical tools to address real-world challenges in quality management. You’ll learn how to implement ISO 15189:2022, optimize workflows, and foster continuous improvement.
SLMTA bridges the gap between theory and practice, with an emphasis on immediate application. You can start improving processes right away. The virtual format offers flexibility, and the facilitators provide expert guidance every step of the way.
This course is more than training—it’s a pathway to excellence that strengthens laboratory services and improves patient care. SLMTA isn’t just recommended—it’s essential!
Kristina Popova
Ukraine
SLMTA 3 eLearning Course – Cohort 5 (2024)
SLMTA is a necessary staple for Quality Managers, Quality Leads and Laboratory Mentors. This course has been true to its tagline “Illuminating the path to ISO 15189”. SLMTA will assist you in guiding laboratories to develop a practical approach to implementing quality management systems. The SLMTA approach is systematic and easy for laboratories with limited resources to apply the quality management principles.
Demetri Smith-Wint
Jamaica
SLMTA 3 eLearning Course – Cohort 5 (2024)
Are you ready to take your laboratory management skills to the next level? Join the SLMTA 3 course and unlock a wealth of knowledge, resources, and expertise designed to empower you in improving laboratory quality and performance. Don't miss the opportunity to transform your laboratory practice and make a lasting impact in the healthcare industry!
Lydia Makarau
Zimbabwe
Congratulations to COHORT 5 WINNERS!

Vladimir Sianghio
Philippines

Nadia Bueno
Argentina

Benefo Ebenezer
Ghana

Pich Sopanha
Cambodia

Robert Reyes
Philippines
The Virtual Incentive Program is designed to promote timely attendance and submission of assignments, quality homework, active class participation, and outstanding contribution to shared learning. Participants earn virtual SLMTA dollars based on criteria. The winners (top earners) are awarded real, not virtual, prizes.
Scientific Committee
Below is a list of Scientific Committee members that contributed to the development of the SLMTA 3 e-learning course:
- Dr. Katy Yao, Centers for Disease Control and Prevention (CDC), USA
- Mr. Ashaba Davis, African Field Epidemiology Network (AFENET), Uganda
- Ms. Anna Murphy, Independent Consultant, USA
- Mr. Elde Mel Paladar, Adventist Health International and Loma Linda University, Malawi
- Ms. Beatrice van der Puije, African Society for Laboratory Medicine (ASLM), Ghana
- Ms. Janet Scholtz, QMS Consultant (Retired from National Health Laboratory Services, South Africa)
- Dr. Luciana Kohatsu, Centers for Disease Control and Prevention (CDC), USA

 Illuminating the Path to ISO 15189 (SLMTA 3) e-Learning Course
Illuminating the Path to ISO 15189 (SLMTA 3) e-Learning Course